Theoretical Study on the Conformational Equilibrium of 1’, 2' and 3'-Nitro-4-hydroxy-3-methoxy Chalcone Isomers
Published 2021-06-30
Keywords
- Conformational Analysis,
- Chalcones,
- Theoretical Calculation,
- NBO
How to Cite
Abstract
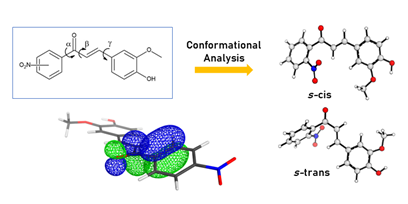
This paper presents the conformational analysis of three chalcone compounds, 1'-nitro-4-hydroxy-3-metoxychalcoea (o-CHAL), 2'-nitro-4-hydroxy-3-metoxychalcone (m-CHAL) and 3'-nitro-4-hydroxiy3-metoxychalcone (p-CHAL). By employing theoretical calculations, the most stable conformers of each compound were obtained. The natural bond orbital (NBO) analyses were carried out with all compounds using the Density Functional Theory (DFT) and the M06-2X method combined with the basis set 6-311++G (2d,dp) in isolated phase and indicated greater electron delocalization in s-cis conformers. The theoretical calculations provided the parameters that led to more stable s-cis conformational equilibrium for all the isomers under investigation. However, the s-trans conformer population was influenced by the position of the nitro group in the ring A, mainly o-Chal.
