Exploring the Selective Generation of Arsenic Hydrides in Quaternary Ammonium Salt Suspension: A Didactic and Systematic Qualitative Approach Applied to Wastewater Treatment
Published 2024-06-24
Keywords
- Analytical Chemistry Learning,
- Arsenic speciation,
- Atomic Spectrometry,
- Reuse of waste,
- Vapor chemical generation
How to Cite
Abstract
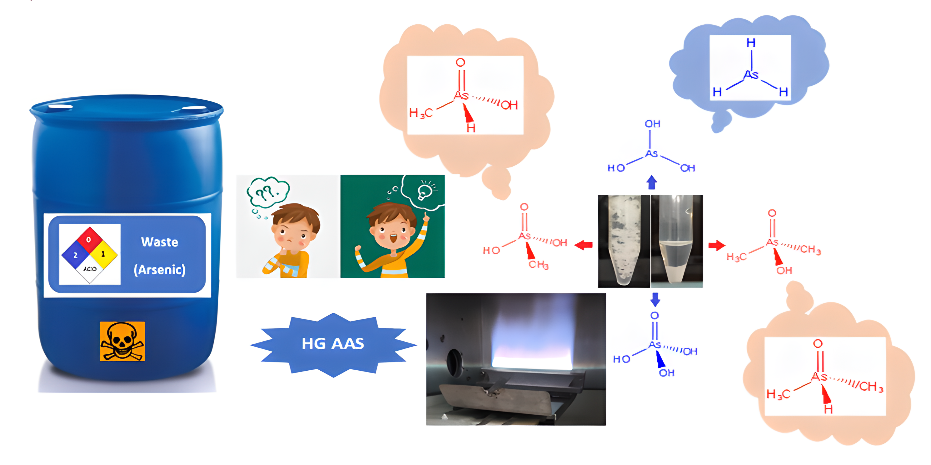
The treatment of laboratory waste containing arsenic compounds should be part of the chemical analysis protocol in educational and research institutions. An alternative procedure for the treatment of these residues is proposed for the development of a didactic analytical route in the detection of As3+, As5+, MMA (monomethylarsonic acid) and DMA (dimethylarsinic acid). The treatment is based on the coprecipitation of inorganic arsenic species with a quaternary ammonium salt (tetra-n-butylammonium perchlorate) in suspension. Detection occurred by controlling the hydride generation reactions and Atomic Absorption Spectrometry. The methylated species MMA and DMA remain in the supernatant and can be monitored by adjusting the pH, concentration of the borohydride reducer, and the use of auxiliary reducers (hydroxylamine hydrochloride and KI). Satisfactory selective detectability was obtained for each arsenic species present in the waste sample. This work contributes to the development of experiments to be implemented in Qualitative Analytical Chemistry and Instrumental Analysis classes. The results allow to approach the chemical vapor generation, which is generally not well explored in undergraduate classes. Discussion of this procedure is supported by a teaching proposal based on the theory of nascent hydrogen for inorganic arsenic species and the nucleophilic performance of the hydride ion for methylated species. This approach allows the student to contextualize important concepts such as Lewis structure, oxidation state and kinetics of arsenic hydride generation reactions. This interdisciplinary contextualization forms a common theoretical basis, preparing and enabling the student to research speciation analysis by hydride generation and to understand the most accepted theories.




