Published 2025-11-23
Keywords
- Adsorption,
- Biosorbent,
- Teak Wood Sawdust,
- Isothermal,
- Kinetic
- Methylene blue ...More
How to Cite
Abstract
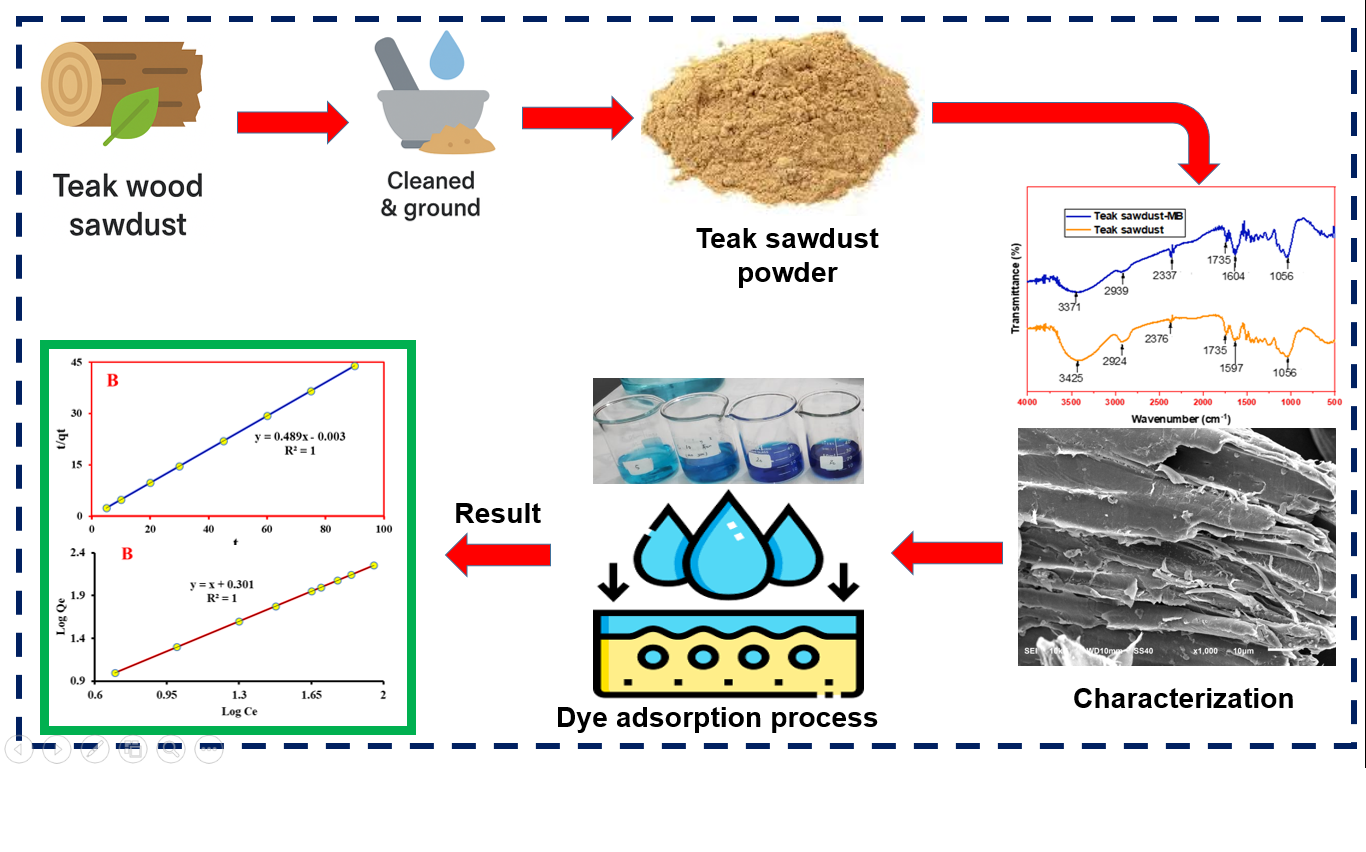
The expansion of the textile industry on a global scale has led to a decline in the quality of our environment due partly to the increasing discharge of untreated textile waste. Industrial textile waste generates two types of environmental pollution-heavy metals and dyes; both possess mutagenic and carcinogenic properties. While efforts have been made to reduce the negative effects of dyes, adsorption remains one of the most cost-effective and effective method. In this context, teak wood sawdust, a readily available byproduct of carpentry activities, was investigated as a potential biosorbent for the removal of methylene blue from aqueous solution. The inherent present of cellulose, hemicellulose, and lignin in teak sawdust make it an ideal biosorbent. The physicochemical properties and surface functionalities of the biosorbent were characterized using Fourier Transform Infrared Spectroscopy (FTIR) and Scanning Electron Microscopy-Energy Dispersive X-ray Spectroscopy (SEM-EDX). This study uses the UV-Vis Spectrophotometer measurement method to determine the effect of pH, contact time, and concentration on methylene blue's adsorption capacity. The optimal conditions for the adsorption were identified at pH 6, with an equilibrium time of 30 minutes and an initial methylene blue concentration of 30 ppm. Under these conditions, the adsorption efficiency reached 99.40%. Kinetic modeling revealed that the adsorption process followed a pseudo-second-order model, with a rate constant of 79.70 g mg⁻¹ min⁻¹. The maximum adsorption capacity was determined to be 1.351 mg g⁻¹, and the process achieved a methylene blue removal efficiency of 99.79%, with a Freundlich isotherm constant (n) value of 1, indicating favorable adsorption characteristics.




