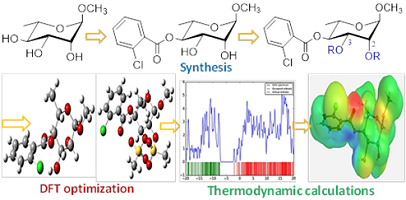
Methyl 4-O-(2-chlorobenzoyl)-α-L-rhamnopyranosides: Synthesis, Characterization, and Thermodynamic Studies
Published 2021-03-30
Keywords
- DFT calculations,
- MEP,
- Methyl α-L-rhamnopyranoside,
- Selective esterification,
- Sugar esters (SEs)
- Thermodynamic calculations ...More
How to Cite
Abstract
Sugar esters (SEs) with promising antimicrobial functionality were found to be a better choice to solve the multidrug resistant (MDR) pathogens due to their improved antimicrobial efficacy, and drug-likeness properties. In this context, 2-chlorobenzoyl ester group at C-4 position of methyl α-L-rhamnopyranoside was prepared via 2,3-O-acetonide protection followed by unimolar 2-chlorobenzoylation, and acetonide deprotection. The selective 4-O-(2-chlorobenzoyl)-α-L-rhamnopyranoside, thus formed, was converted into five 2,3-di-O-acyl esters with different aliphatic, and sulphonyl chains to obtain biologically important novel rhamnopyranoside-based SEs. All the synthesized compounds were optimized employing density functional theory (DFT). Thermodynamic calculations including frontier molecular orbital, and molecular electrostatic potential (MEP) were calculated and discussed. Attachment of multiple ester groups enhanced their stability, reactivity, and softness indicating their more polar and reactive nature than the non-ester sugars. Corroboration of all these properties might be helpful for their interactions with several enzymes (proteins) during different biological activities. The present study also revealed that incorporation of 2-chlorobenzoyl and mesyl groups in rhamnopyranoside skeleton increased better thermodynamic properties.
