Basic Ionic Liquid [Bmim]OH as Efficient Greener Medium for the Oxidation α-Hydroxy Ketone Compounds and Alcohols to Carbonyl Compounds with Potassium Permanganate
Published 2022-12-26
Keywords
- Alcohol,
- [Bmim]OH,
- 1,2-Diarylethane-1,2-dione,
- α-Hydroxy ketone,
- Ionic liquid
- Oxidation ...More
How to Cite
Abstract
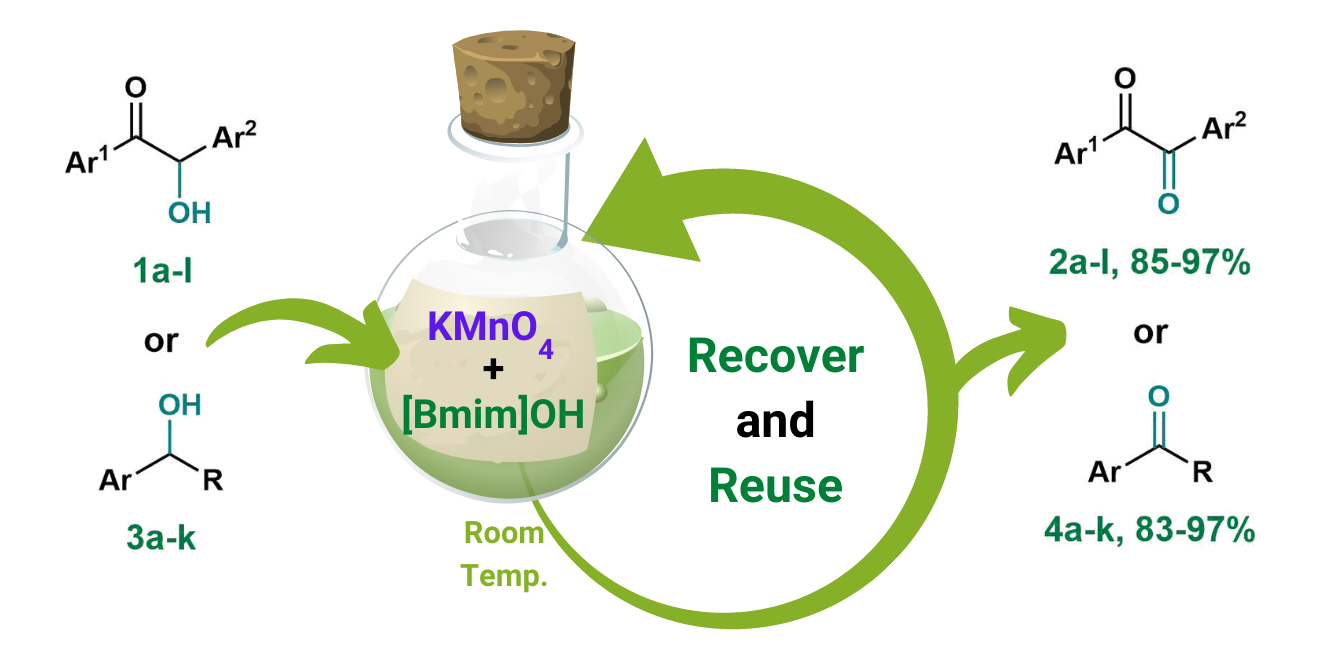
In this work, a convenient and greener procedure for synthesizing 1,2-diarylethane-1,2-dione derivatives as well as the oxidation of primary and secondary alcohols was explored using an oxidizing agent (KMnO4) in a basic ionic liquid of 1-butyl-3-methylimidazolium hydroxide ([Bmim]OH) as greener medium. The selective oxidation of α-hydroxy ketone compounds together with primary and secondary alcohols in the presence of KMnO4 in [Bmim]OH afforded corresponding carbonyl compounds of 1,2-diarylethane-1,2-dione derivatives and aldehydes or ketones in high yields (85-97% and 83-97%, respectively). The reactions are mild, fast, and efficient. Moreover, KMnO4 in [Bmim]OH medium was easily recovered and reused for at least four additional reactions without significant loss of efficiency with a consistent yield of about 80%.




