1,2-O-Isopropylidene-3-O-butylglucofuranose-derived Ester, and Ether: Synthesis, Characterization, and Antimicrobial Study
Published 2023-07-13
Keywords
- Antimicrobials,
- Glucofuranose,
- PASS analysis,
- Sugar fatty acid esters,
- Zone of inhibition
How to Cite
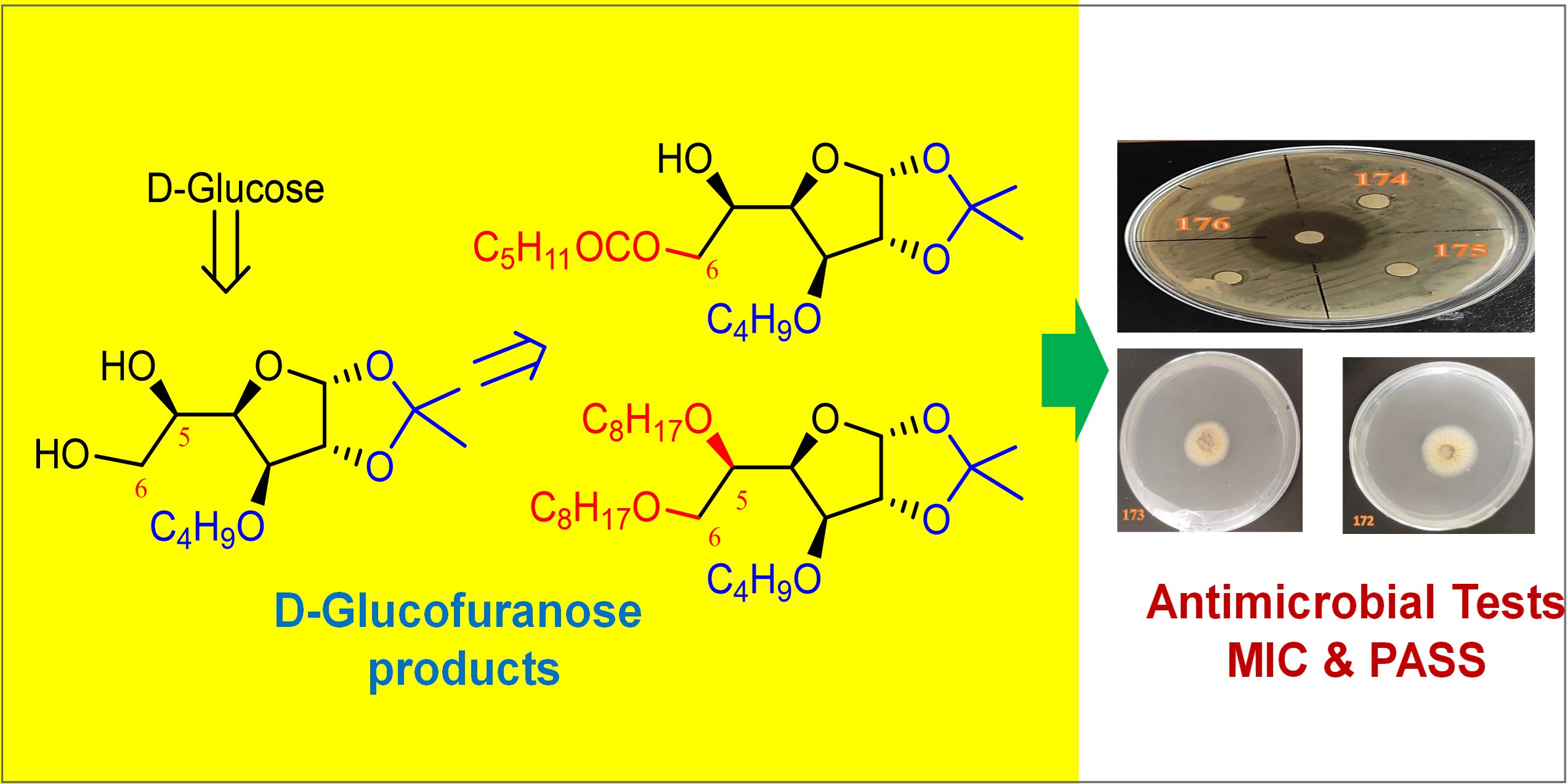
Abstract
Sugar-fatty acid esters (SFAEs, especially sucrose- and glucose-based ones) have dominated the chemical industries for more than 50 years. In comparison to other carbohydrate products, SFAEs serve essential roles in a variety of industries, including the food, pharmaceutical, and cosmetic industries. In this context, the 6-O-hexanoyl ester of 3-O-butyl-1,2-O-isopropylidene-α-D-glucofuranose was synthesized from commercially available D-glucose in a few steps. For a comparative biological study, the 5,6-di-O-benzyl ether of 3-O-butyl-1,2-O-isopropylidene-α-D-glucofuranose was also prepared and characterized. An in vitro antimicrobial test of all the ester and ether compounds indicated that these compounds are more susceptible to fungi than bacteria. Also, they have more potential for A. niger than A. flavus. According to Prediction of Spectra for Substances (PASS), the chemicals found in the current investigation have a variety of potential biological functions.




