Novel Piperidone Hydrazine Carbodithioate Derivative: Synthesis, In Silico Drug-Likeness Analysis and Anticancer Properties
Published 2024-09-27
Keywords
- Anticancer, Drug likeness, Hep G2 cell lines, Hydrazine carbodithioate, Piperidone, SwissADME
How to Cite
Abstract
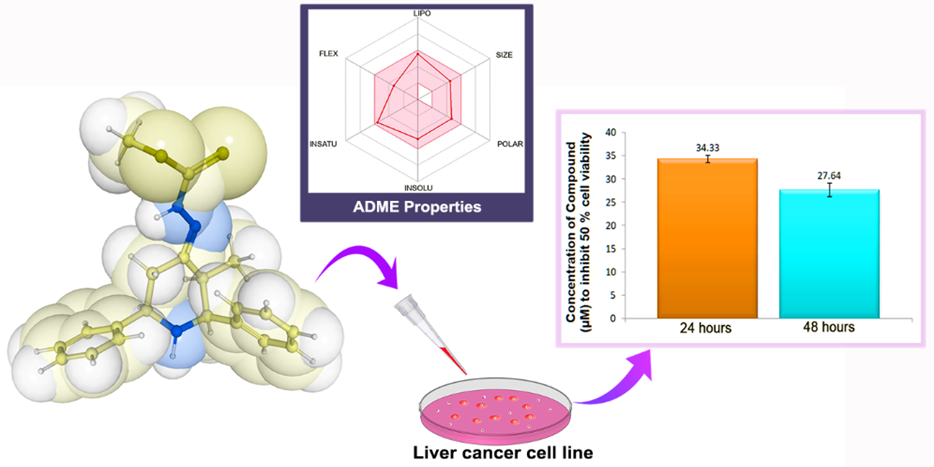
An efficient synthesis of a novel compound of hydrazine carbodithioate derivative of piperidone, (E)-methyl-2-(3-methyl-2,6-diphenylpiperidin-4-ylidene)hydrazinecarbodithioate (1), was performed using methyl dithiocarbazinate and 3-methyl-2,6-diphenylpiperidin-4-one as the starting materials. The reaction was carried out in an acidic medium using methanol as a solvent. The novel compound was characterized by FTIR, Mass, and NMR spectral techniques. The hydrazine carbodithioate derivative (1) was then tested for its anticancer activity against the liver cancer cell line, Hep G2, using the MTT assay. The IC50 values of the newly synthesized compound were found to be 34.33 ± 0.79 µM (24 hours) and 27.64 ± 1.42 µM (48 hours). The in vitro antitumor studies demonstrated that the novel compound (1) exhibited good inhibitory activity against the Hep G2 cancer cells. Furthermore, in silico properties such as lipophilicity, water solubility, pharmacokinetic properties, drug likeness, and medicinal chemistry were analyzed using the SwissADME tool.




