Polyimides Derived from Diglycidyl Ether of Bisphenol-A and Bisphenol-F via Diels–Alder Polymerization: Polyimides
Published 2025-05-02
Keywords
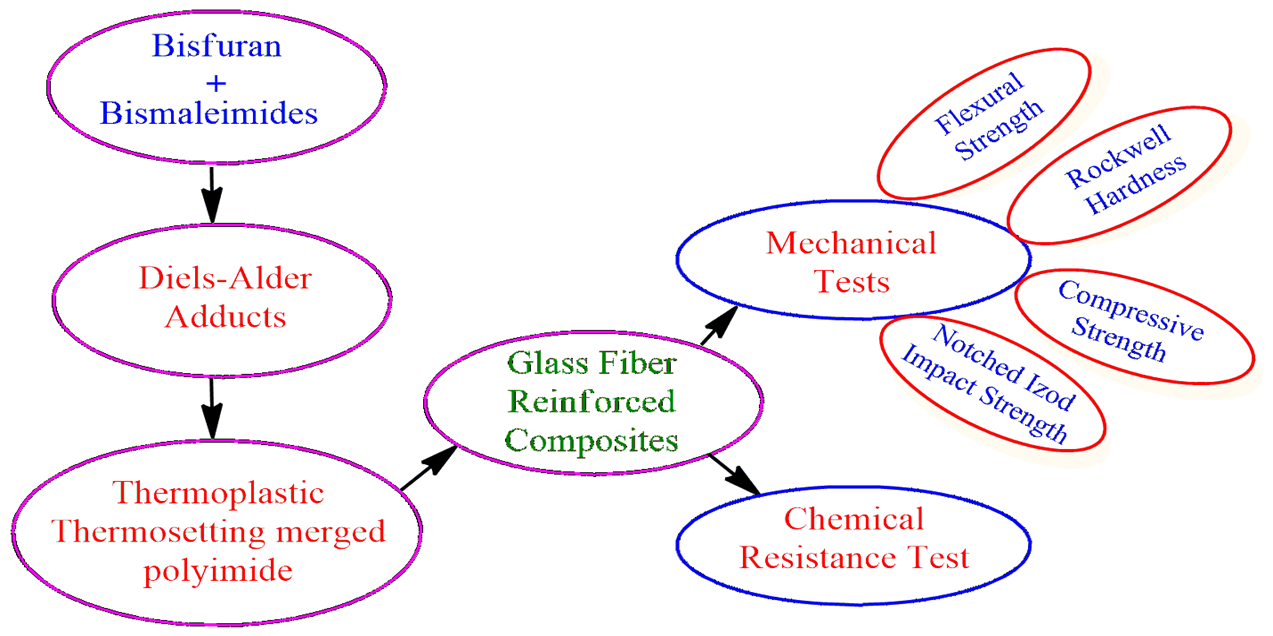
- Bisfuran,
- Bismaleimide,
- Diels-Alder reaction,
- Polyimide
How to Cite
Abstract
Novel polyimides were synthesized by Diels–Alder polymerization. Bisfuran (BF) was reacted with several bismaleimides containing the diglycidyl ether of bisphenol-A and bisphenol-F (epoxy segments) to obtain Diels–Alder polyadducts. These polyadducts were subsequently aromatized and imidized (i.e., cyclized) through carboxylic acid and amide group formation to yield polyimides. The synthesized polyadducts and polyimides were characterized by elemental analysis, spectral techniques, number average molecular weight (Mn), and thermal analysis. The ‘in situ’ glass fiber-reinforced composites were prepared and characterized for their mechanical, electrical, and chemical properties. These properties were compared with those of other reported polyimides. All the composites exhibited good mechanical and electrical properties, as well as excellent resistance to organic solvents and mineral acids.




