Published 2025-04-28
Keywords
- Ammonium nitrate,
- Copper,
- Lead, Liquid,
- Silver
How to Cite
Abstract
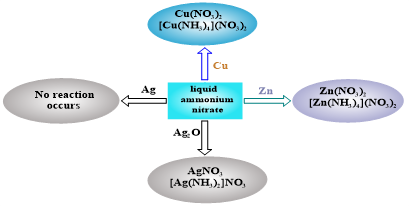
Ammonium nitrate salt has been known for a long time. Many studies have been conducted to determine its properties. Paying attention to the results obtained so far, we conducted research on the reaction of copper, zinc, lead, silver and silver(I) oxide substances in the liquid medium of ammonium nitrate and to study its causes. The conducted research showed that only when metals are oxidized in liquid ammonium nitrate, they can react and form nitrate salts. The fact that silver metal did not react in liquid ammonium nitrate showed that metals cannot react with ammonium nitrate without oxidation. The possibilities of reacting metals with ammonium nitrate were studied using quantum chemical calculations. The results of the calculations helped to prove the correctness of the research conclusions. These reactions can serve to reveal new properties of ammonium nitrate salt.




