Evaluation of the Quality Parameters of Anti-flu Combinations Containing Phenylephrine Hydrochloride Available in the Brazilian Market
Published 2025-11-25
Keywords
- Anti-flu drugs,
- Impurity profile,
- Michael addition,
- Phenylephrine hydrochloride,
- Quality assessment
How to Cite
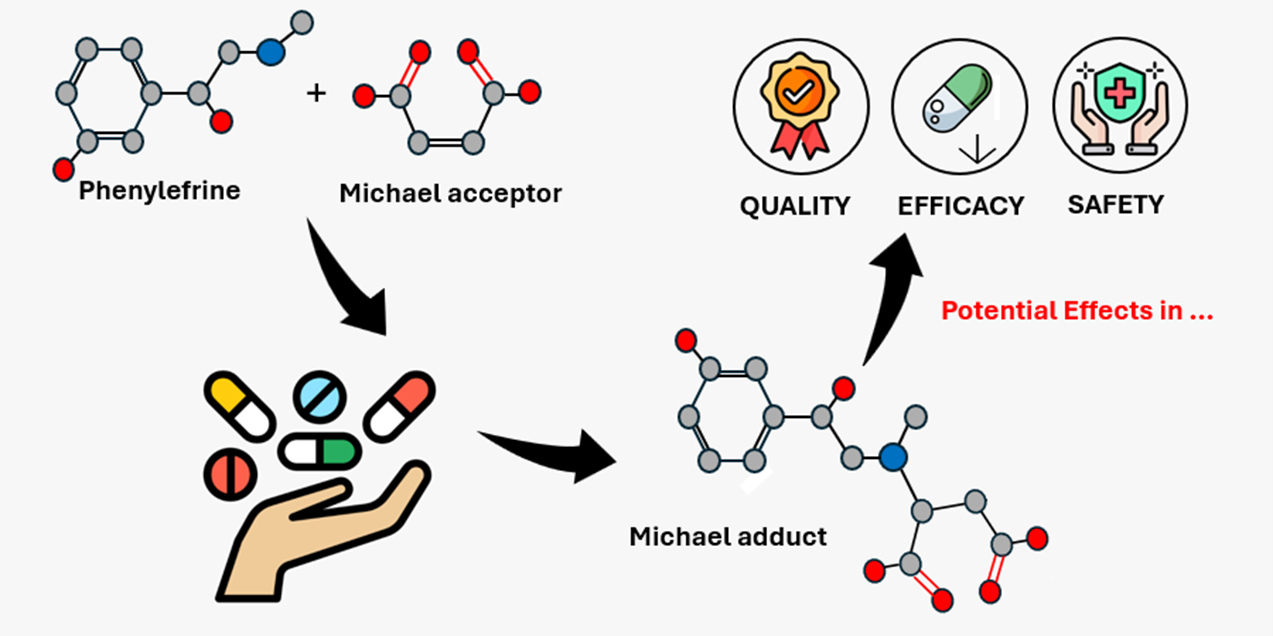
Abstract
The combination of different active substances in drug products is responsible for optimizing therapies and improving patient adherence. However, the development process of these formulations is highly challenging due to differences in the physicochemical properties of the active ingredients, which can impact formulation stability. Thus, the present study evaluated the incompatibility profile in five different combinations/formulations containing phenylephrine hydrochloride and the maleate compound as a counter-ion by measuring the active pharmaceutical ingredient (API) content and the degradation products (succinyl phenylephrine adducts). This incompatibility occurs due to reactions via Michael addition. The quantification of the API and degradation products was performed using an HPLC-DAD system. The analyses revealed that in all formulations where there is no physical separation between phenylephrine and the maleate counter-ion, a decrease in phenylephrine content and appearance of impurities were observed. Furthermore, the physical separation between the active ingredients was effective, and no markers of the respective reaction were identified. Based on these findings, it is evident that the pharmaceutical form impacts formulation stability, and the reduction in API content along with the increase in impurities may negatively affect product efficacy, quality and safety parameters. This reinforces the need for additional information regarding regulatory registration aspects.




