Published 2021-07-06
Keywords
- Crystal structure,
- Powder X-ray diffraction,
- Rietveld method,
- Semiconductor
How to Cite
Abstract
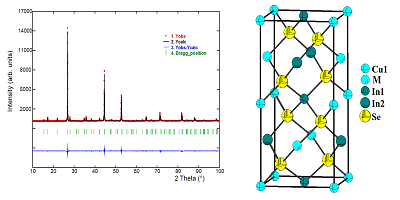
This work focuses on the preparation and structural characterization of the semiconductor Cu3In5Se9, an important member of ordered vacancy compounds family, belonging to the semiconductor system I3-III5--VI9, where denotes the cation vacancy which is included in the formula to maintain the same number of cations and anions sites. This material was synthesized by the Bridgman-Stockbarger technique, and its structure was refined from powder X-ray diffraction data using the Rietveld method. Cu3In5Se9 crystallizes with tetragonal symmetry in the space group P2c (Nº 112), with a = 5.7657(1) Å, c = 11.5353(4) Å, V = 383.47(2) Å3. This ternary compound consists of a three-dimensional arrangement of distorted CuSe4 and InSe4 tetrahedral connected by common faces. In this structure, each Se atom is coordinated by four cations located at the corners of a slightly distorted tetrahedron, and each cation is tetrahedrally bonded to four anions. Cu3In5Se9 is related to the p-type CuInSe2 and n-type CuIn3Se5 semiconductor compounds, which are being used in the preparation of high-efficiency solar cells.
