Published 2021-07-06
Keywords
- Acylation,
- Antimicrobial agent,
- Benzyl α-L-rhamnopyranoside,
- Conformational study,
- HOMO-LUMO
- Thermodynamic calculations ...More
How to Cite
Abstract
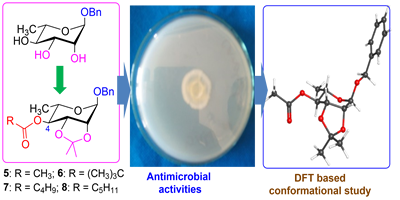
Application of carbohydrate fatty acid (CFA) esters in food and beverage industries has increased their interest in other fields. Especially rhamnopyranoside esters having both the hydrophilic and lipophilic nature had broader applications including anticancer activities. Benzyl α-L-rhamnopyranoside, prepared from L-rhamnose, on 2,3-O-isopropylidene protection with 2,2-dimethoxypropane followed by acylation at C-4 hydroxyl position with different acylating agents furnished the corresponding 4-O-acyl-α-L-rhamnopyranosides in good yields. All the compounds were well characterized by spectroscopic techniques. In vitro antimicrobial activities against eight bacterial and two fungal pathogens indicated that these 2,3-O-isopropylidene protected rhamnopyranosides had weak to moderate inhibitory properties. To rationalize such moderate activities structural (conformational) distortion of these monoacetonide protected CFA esters were studied from the density functional theory (DFT) optimized structures. In addition, thermodynamic properties including frontier molecular orbitals of the synthesized rhamnopyranosides were calculated and discussed. Corroboration of all the studies signifies that the moderate antimicrobial efficacy of the isopropylidene protected rhamnopyranosides might be due to their distorted conformations, lower softness and smaller dipole moments.
