Green Chemistry Approach to Corrosion Inhibition of Mild Steel in Acidic Medium Using Myrica esculenta Extract
Published 2025-12-06
Keywords
- Myrica Esculenta,
- Green corrosion inhibition,
- Electrochemical analysis,
- Adsorption isotherms,
- Protective coating
- Polarization studies ...More
How to Cite
Abstract
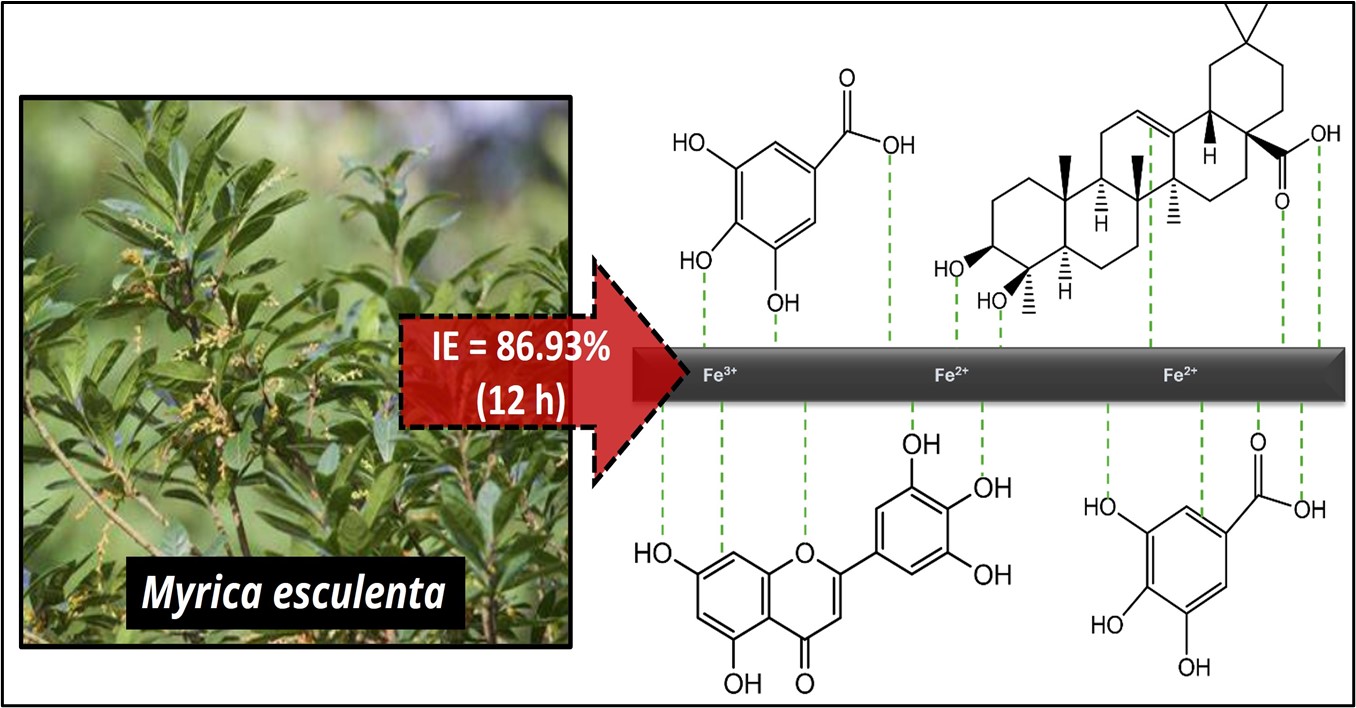
Corrosion of mild steel (MS) in acidic environments presents a significant challenge in industrial applications, often leading to structural degradation and economic losses. This study investigated the effectiveness of Myrica esculenta (ME) extract as a green corrosion inhibitor for MS in acidic media. A combination of methods—including weight loss experiments, contact angle measurements, surface analysis, and electrochemical techniques—was employed to evaluate the inhibitor's performance. In the absence of the ME inhibitor, MS experienced rapid corrosion and significant weight loss. However, the addition of ME extract, particularly at a concentration of 40 mg/L, markedly reduced corrosion, achieving an inhibition efficiency of 86.93% after 12 hours. Electrochemical polarization studies revealed a shift in corrosion potential toward more noble values, indicating a reduced tendency for corrosion. Adsorption studies suggested that the inhibitor formed a protective layer through both monolayer and possible multilayer adsorption mechanisms. Contact angle analysis showed an increase in surface hydrophobicity, confirming the formation of a protective interface layer. UV analysis further validated the presence of this layer, which played a crucial role in minimizing pitting and preserving the structural integrity of the mild steel surface. These findings underscore the potential of ME as an effective and environmentally benign corrosion inhibitor.




