Synthesis and Characterization of Novel Rhenium Tricarbonyl Complexes with Dipicolylamine Sulfonamide Ligands: Potential Serine/threonine Kinase Inhibitors for Cancer Therapy
Published 2026-02-19
Keywords
- Cancer drugs,
- Dipicolylamine sulfonamide ligands,
- Kinase inhibitors,
- Rhenium tricarbonyl complexes
Copyright (c) 2026 Orbital: The Electronic Journal of Chemistry

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
Abstract
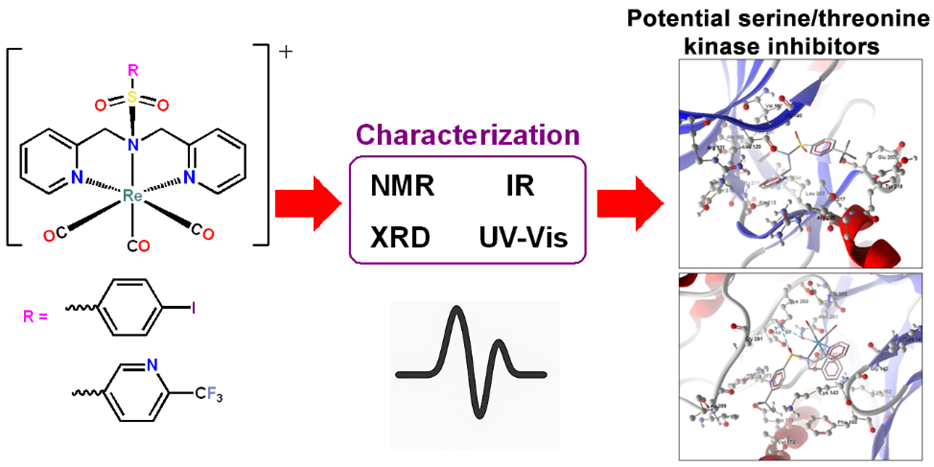
This study explores the synthesis of rhenium complexes using two novel ligands; N(SO2(Iodobenz))dpa (L1) and N(SO2(tfm)py)dpa (L2). The formation of these ligands and their corresponding rhenium complexes, [Re(CO)3(L1)]⁺ (C1) and [Re(CO)3(L2)]⁺ (C2), was meticulously confirmed through 1H and 13C NMR spectroscopic analysis. Interestingly, the singlet peak associated with the methylene hydrogens in the ligands (4.54 ppm for L1 and 4.66 ppm for L2) transformed into two doublets in the complexes, indicating distinct magnetic environments upon complexation with rhenium. Structural verification via single-crystal diffraction confirmed the successful synthesis of L1, L2, C1 and C2. UV-Visible spectroscopic analysis aided in the identification of π−π* transitions within the 200-265 nm range for ligands, and MLCT (metal-to-ligand charge transfer) transitions around 310 nm for the complexes. FTIR spectra exhibited significant changes in the S-N stretching bands of L1 (916 cm-1) and L2 (919 cm-1) upon coordinating to Re (C1 (832 cm-1) and C2 (830 cm-1). While both ligands displayed considerable fluorescence, both complexes showed quenched fluorescence. Swiss TargetPrediction analysis indicated the potential of these compounds as Serine/Threonine protein kinase inhibitors. Molecular docking studies of L1, L2, C1 and C2 with human Serine/Threonine protein kinase Aurora A and PIM2 revealed favorable binding affinities and emphasize their potential as lead compounds in the cancer drug development.




